Zirconium is a chemical element with the symbol Zr and atomic number 40. It is a lustrous, gray-white, strong transition metal that resembles titanium. Zirconium is used as an alloying agent due to its high resistance to corrosion. It is never found as a native metal, but is instead obtained mainly from the mineral zircon, which can be purified by chlorine. Zirconium was first isolated in an impure form in 1824 by Jöns Jakob Berzelius.
Zirconium has no known biological role. Zirconium forms both inorganic and organic compounds, such as zirconium dioxide and zirconocene dibromide, respectively. There are five naturally-occurring isotopes, three of which are stable.
Zirconium is a lustrous, grayish-white, soft, ductile, and malleable metal which is solid at room temperature,though it becomes hard and brittle at lower purities.
In powder form, zirconium is highly flammable, but the solid form is far less prone to igniting. Zirconium is highly resistant to corrosion by alkalis, acids, salt water, and other agents. However, it will dissolve in hydrochloric and sulfuric acid, especially when fluorine is present. Alloys with zinc become magnetic below 35 K.
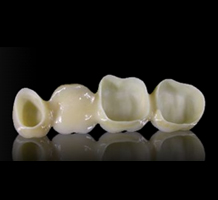
Crowns and bridges
Zirconia is an innovative system for esthetic metal-free crowns and bridges. The reliability of zircon restorations is backed by more than five years of proven clinical success. The system is used to fabricate anterior or posterior single units or multi-unit bridge restorations out of translucent, biocompatible zirconia, the strongest and toughest dental ceramic.
Milled crown made of zircon crystal or so-called zircon crown is the most modern solution of tooth-crowning. Also this solution includes a basic construction that is milled of zircon crystal and it is then covered with plastic porcelain. Advantage of this method is the fact that zircon ceramic is very firm and it can be applied to more serious dentition defects, i.e. loss of 3-4 neighbouring teeth.